親,你被這個標題嚇到了嗎?
這是幾個月前,美國FDA給大森林發來的一段留言。沒嚇到各位客戶,倒是讓大森林人嚇到魂飛魄散。
2017年11月,大森林拼箱45HQ前往美國FTW1倉庫,該柜中大部分貨物需要做FDA REVIEW( FDA只是審核并不強制要求做FDA注冊的貨物),及另外一部分LED產品。
在貨物到港之前,大森林美國報關行進行清關及將FDA REVIEW貨物進行FDA申報。海關在接收到清關資料后24小時內給予放行,而由于有FDA監管貨物,FDA部門暫時沒有對他管轄內的貨物放行,并向大森林報關行發送了一份電子HOLD貨通知書:


此說明書說明的清清楚楚,
只有STAINLESS STELL FOOD SCISSORS
CUPPING THERAPY
MANUAL HANDHELD MASSANGER
BODY MASSANGER
貨物需要等待FDA REVIEW
在收到此份通知書后,大森林海外倉迅速作出反應:將貨物進行分揀,將FDA HOLD貨物調出,其他放行貨物先安排預約派送至FBA ,并等候FDA部門對上續四種產品進行審核,一般情況下,FDA會做文件審核后直接放行,另外一種為FDA會到貨物存放現場驗貨后放行。
FDA官大人于1月6日來到大森林代理倉庫進行驗貨。然而驗貨結束后,我們收到這樣一份通知(一封來自大森林美國報關行的郵件):
LED’s were not at the facility and available for FDA exam. LED’s which are FDA regulated products must be transmitted with all future entries They advised that if we can request that the customer locate these goods and make them available for exam, if possible.
Also, they asked that we provide the actual manufacturer’s name and complete address for each FDA regulated items also if they have facility registration numbers also include them with our response.
So that we may know exactly what LED is, as it is too broad a term, please advise what this items is and please make sure invoices are detailed as when vague, it is causing too many problems
FDA查貨現場居然聲稱為什么沒有LED產品?
LED產品去哪里了?
I have detained 4 lines of this entry and will forward to our Center for Devices for review. However, there appears to be differing manufacturer information. The entry documents have one firm name, but the cartons have the name of Yongkang Jinkang Health Equipment Factory. I need to know as soon as possible the actual manufacturer of these devices. If the manufacturer is Yongkang, the Importer will need to provide an invoice from that manufacturer. If they are not, please provide an explanation as to why Yongkang is on the product labeling and outer cartons.
這封來自FDA的郵件,FDA聲稱產品的資料上提供的制造商和產品外箱制造商不符。 ( 這點大森林提議所有客戶,特別是代理客戶,一定如實申報美國制造商)
我們姑且忽略第二個FDA小問題,但第一個問題則讓大森林人抓頭皮了:在FDA扣貨通知書上,哪個單詞看到了LED呢? 拿望遠鏡也看不到LED啊。
但是官大神的話如同圣旨,在收到該郵件通知后,大森林火速通知客戶下架LED產品,通通撤回到大森林代理倉庫,進行最后的檢驗。想想這浩浩蕩蕩的退貨,太雄偉了。(頓時也有種FDA官大人亂用私權的趕腳啊)
由于大批量退貨導致客戶產生巨額退貨費,大森林面臨來自客戶沉重的壓力。于是我們又開始了和FDA部門漫長的談判過程。
溝通了無數個來回無果,FDA最后發來這么一段英文解釋。
如下是來自FDA海關原始郵件配上大森林的翻譯(順帶讓大家體會純正的美國英語):
LED lights are FDA regulated:(LED燈屬FDA監管)
“LED products emit visible optical radiation which qualifies them to be a radiation emitting electronic product and unless it contains a laser, these LED products are not subject to a mandatory standard but the FDA does regulate them.
LED產品發射可見光輻射,使其成為輻射電子產品,除非它含有激光,這些LED產品不受強制性標準,但FDA對它們進行規范監管。
We recommend the manufacturer/importer to claim any product that we regulate and since we regulate all radiation emitting electronic products under the general/defect clause they should all be claimed. The LED manufacturers are subject to the ARO reporting requirement (21 CFR 1002.20) and notification if they discover a defect (21 CFR 1003). The product code we recommend the firms to transmit is shown below;
Since LEDs do not have a performance standard, a 2877 Form shouldn’t be required but you may still need to enter an Affirmation of Compliance of RA2 and state “There is no performance standard”.
我們要求制造商/進口商向我們申報任何屬于我們規管的產品,因為根據一般/缺陷條款,我們規管的所有輻射的電子產品都應該申報。
LED廠商,如果發現一個缺陷(21 CFR 1003),受ARO報告要求(21 CFR 1002.20)和通知管制。我們建議公司使用的產品代碼如下所示;
由于LED沒有性能標準,可能不需要填寫2788表格,但你可能依然需要填寫一份表格,An Affirmation of Compliance of RA2 并聲明“沒有性能標準”。
ORA imports product code is:
ORA進口產品編碼
非裝置發光產品: LED, 照明,一般光學產品,非醫療用, 編碼:95R--HH
Here is additional information on LED product code taken off our website:
下面是從我們網站上下載的關于LED產品的附加信息
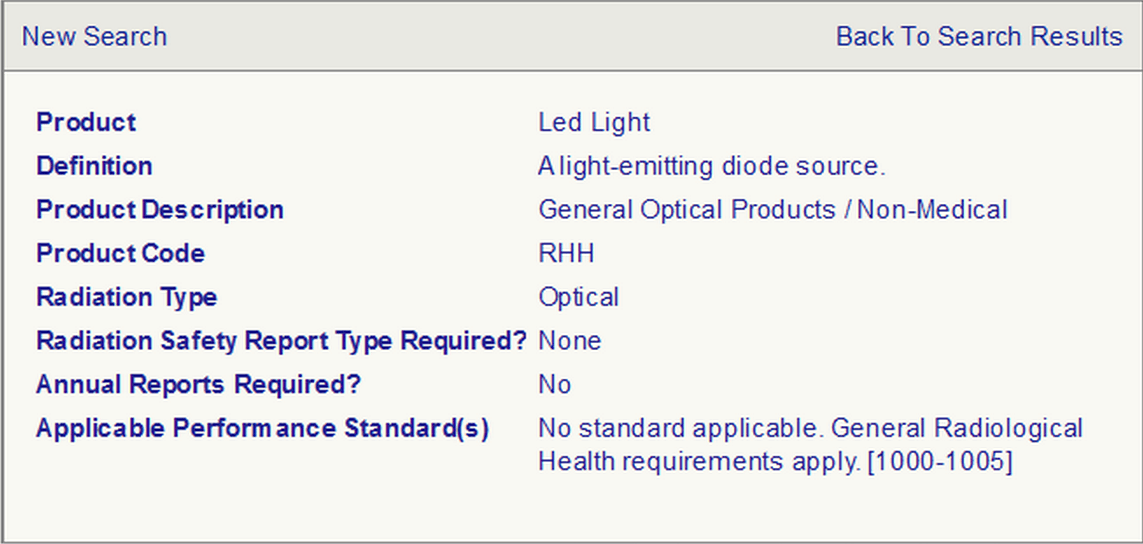
產品:LED 燈
定義:發光二極管光源
產品描述:一般光學產品/非醫療用
產品編碼:RHH
輻射類型:光
是否需要輻射安全報告? 不需要
是否需要年度報告? 不需要
適用的性能標準:無適用標準。一般放射衛生要求(1000-1005)
Also, check out our website where it discusses LEDs: 可以查看FDA網站上關于論述LED產品的信息:
http://www.fda.gov/Radiation-EmittingProducts/ElectronicProductRadiationControlProgram/GettingaProducttoMarket/default.htm
Product Specific Questions
Q35) Are Light Emitting Diodes (LEDs) or Intense Pulsed Lights (IPLs) subject to the laser regulations and reporting?
LEDs and IPLs do not meet the definition of a laser, namely:
21 CFR 1040.10(b)(19) Laser means any device that can be made to produce or amplify electromagnetic radiation at wavelengths greater than 250 nm but less than or equal to 13,000 nm or, after August 20, 1986, at wavelengths equal to or greater than 180 nm but less than or equal to 1.0*106nm primarily by the process of controlled stimulated emission.
There is no existing FDA performance standard for LED or IPL products. They are not subject to Product or Annual reports under 21 CFR 1002. However, as they are radiation-emitting products, the manufacturers of these products would still be subject to the general requirements in Title 21 CFR 1000 through 1005, specifically, accidental radiation occurrence notifications and notifications of defect, 21 CFR 1003 & 1004.
產品具體問題
Q35)發光二極管(LEDs)或強脈沖光(IPLs)是否受激光法規和報告管制?
LEDs和IPLs不滿足激光的定義,即:
21 CFR 1040.10(b)(19)激光設備,能夠產生或放大電磁輻射的波長大于250 nm,但小于或等于13000納米,1986年8月20日后,主要通過控制激光發射過程,在波長等于或大于180 nm,但小于或等于1.0* 106nm。
對于LED或者IPL產品,FDA沒有現存的性能標準。根據21 CFR 1002,他們不受產品報告或年度報告管制。然而,因為它們是輻射發光產品,這些產品的制造商仍然受到21 CFR 1000的一般要求管制,具體來說,通過1005,做出意外輻射發生的通知和缺陷的通知,21 CFR 1003和。
如上美國FDA郵件以及大森林面臨的這次退倉事件證明,最近傳的沸沸揚揚的LED燈被FDA管制,總算有了終極答案: 只有用于醫療設備,帶有激光輻射性的LED燈才會被FDA強制管制,而普通的LED燈并不是不被管制,而是FDA需要做REVIEW(審核),這意味著LED貨物都需要進行FDA申報才能清關放行。也表示報關行要開始收FDA申報費了。
最后的最后,大森林強烈建議:為了減少不必要的麻煩和查驗,LED產品在發票品名需注明 *For Dental Use* *For Industrial Use* *For Medical Use*(牙科用,工業用,醫療用。)這個非常重要,直接決定LED產品是只需要FDA申報還是需要FDA注冊號。
當然,所有貨物也務必真實提供制造商名稱。
更多亞馬遜報價及咨詢,請關注大森林官方服務號:
